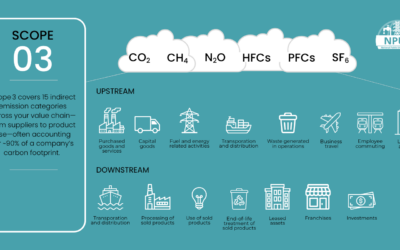
Commercialization
Commercialization is the process of bringing new products or services to customers. In Health & Life Sciences, R&D, or research and development, is a large part of the commercialization process. R&D costs required to successfully bring a new drug to market can easily reach billions of dollars. These costs result from multiple years of highly regulated processes involving dozens of different stakeholder groups, and often do not result in a successful Clinical Trial.
Pharmaceutical and medical device companies are then understandably under extreme pressure to ensure those products that do receive FDA approval are marketed and profitable as soon as possible. It is during these initial years of entry into the market without potential competition that a company can generate massive profits to be able to recoup the costs of other failed R&D efforts and be able to fund future R&D projects.
That is why a focus on Project & Program Management, as well as Change Management, during the Commercialization process is critical. To minimize costly delays and to better ensure all stakeholders are aware and aligned with objectives, specific activities should be considered throughout the process of delivering products to market.
Project & Program Management:
The sheer number of separate activities that have cross-functional dependencies and disparate completion timelines require an integrated schedule to be managed. Project and Program Managers should develop and manage this integrated schedule with inputs from the different stakeholder groups, highlighting the need for constant communication throughout.
This integrated schedule should be resource loaded to understand who from each stakeholder group is available to complete required tasks, for example:
This resource loaded schedule will showcase the need for collaboration across
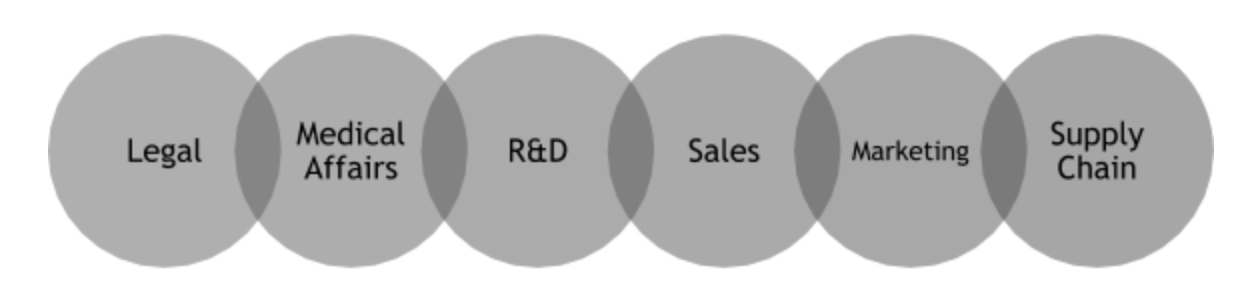
these groups reducing the likelihood of information silos that cause issues with hitting deadlines and inconsistent knowledge across the program. Additionally, it will highlight gaps to the intended resource plan and provide the maximum amount of time to identify and obtain external contracted talent as necessary.
For Commercialization within pharmaceuticals, typical activities need to focus on a range of outcomes, including how patients will be prescribed the drug, whether additional services should be provided based on expected disease stages, competition research that will lead to price point decisions, and more. Stakeholders can provide updates on their individual required tasks to meet the defined deliverables and outcomes.
Risk management will also play an important role in any holistic Project & Program Management undertaking for improved Commercialization. An evaluation of what potential issues could delay schedules or cause additional budget expenditures should be evaluated as early in the effort as possible and then reviewed weekly. Some examples of categories to be evaluated for potential risks that will need to be mitigated are below.
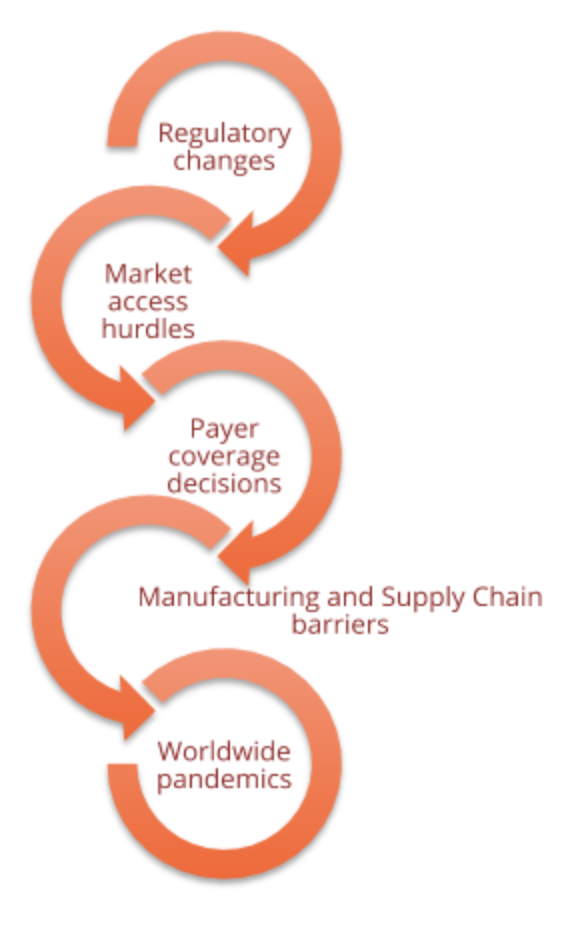
Identifying with stakeholders what specific risks could affect each activity, what the financial and timeline impacts would be, and what actions the team will take once the risk triggers. By taking the time beforehand to discuss the plan of attack, more options are available to reduce those impacts.
Companies can create a standardized, repeatable process flow that contains all necessary forms, documentation, and guidance within each process step. This standardization of a commercialization process will reduce delays based on utilizing out of date forms, as well as ensure that decisions are made at the lowest level authorized (RACI).
Motive Power has assisted companies to visualize and operationalize standardized processes through their Process Optimization Platform (PoP) capability.
Change Management:
Commercialization of a product introduces change within both the internal team and external stakeholder groups to include patients, payers, supply chain companies, etc. This change will affect individuals & organizations differently and each individual will transition from the current state to the desired future state at different times, if ever.
Focusing time and resources to ensure this transition from current to future states occur as quickly as possible will reduce the risk that speed to market profitability isn’t delayed. Even the best project management plan could be implemented but will have no effect if the individual stakeholders expected to change behaviors to make the commercialization effort successful, don’t change.
Furthermore, efforts to analyze the impact of the new product on each stakeholder organization will help to assess the level of change management required. If the new product radically shifts how a physician needs to prescribe a product, then additional training, targeted messaging, and follow-up should be planned and implemented. Change adoption metrics should be developed and implemented to gain insights as early as possible when individual stakeholders are resistant to the requested change. Effective change management is paramount to any project’s success.
For more information on how Motive Power can assist your Commercialization effort, please contact us at sales@motive-power.com.
Author: Nick Bruno